Explain Why We Must Balance All Chemical Equations
A balanced equation obeys the Law of Conservation of Mass. Writing balanced chemical equations is essential for chemistry classHere are examples of balanced equations you can review or use for homework.
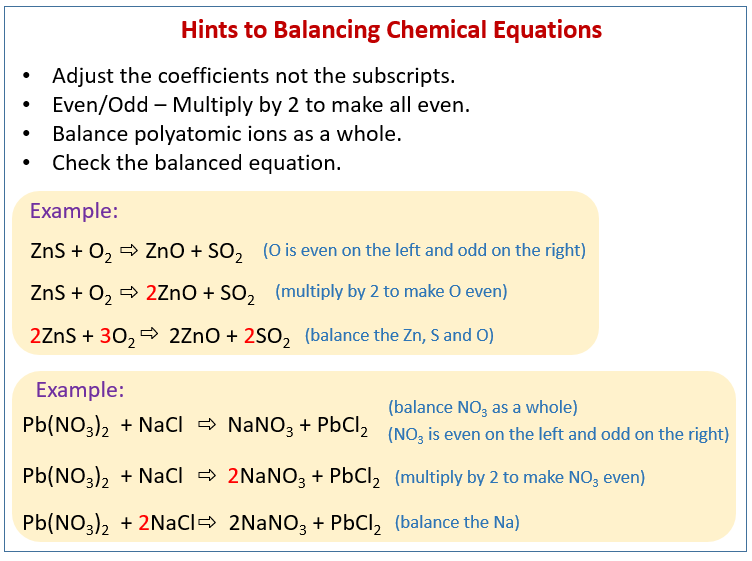
Balance Chemical Equations Solutions Examples Videos
Steps of Balancing a Chemical Equation.

. What happens if a chemical equation is not balanced. The chemical equation needs to be balanced so that it follows the law of conservation of mass. A chemical equation must be balanced because the law of conservation of mass says that mass can neither be created or destroyed.
This chemical law states that in order for the equation to be correct An equal quantity of matter exists both before and after the experiment. Knowing the skeletal reaction equation we know what the reactants are and what are the products but for quantitative predictions we need to balance the reaction equation. A balanced chemical equation occurs when the number of the different atoms of elements in the reactants side is equal to that of the products side.
Chemical equations need to be balanced in order to satisfy the law of conservation of matter which states that in a closed system matter is neither created nor destroyed. The mass of the reactants must equal the mass of the products. The law states that mass cannot be created and destroyed as well.
The debit side of the transaction is already accounted for correctly so the amount of assets dont need to change. To balance the accounting equation we need to credit the income account twice. Laws in science are some rules applied on every form of matter.
According to this law mass can neither be created nor be destroyed in a chemical reaction and obeying this law the total. This is an important guiding principal in science. When two things are mixed then we get a new thing.
To establish the mole relationships needed for stoichiormetric calculation. If an equation is unbalanced you are effectively showing atoms vanishing or appearing from nowhere. This would violate the law of conservation of mass.
Reaction balancing is based on mass preservation. Scientists have found through thousands of experiments that in a chemical reaction mass is conserved which means that if we start with ten grams of reactants starting materials we must end. Atoms are never lost or gained in chemical reactions they are rearranged.
This is called balancing of the chemical equation. This is necessary because any chemical equation must obey the law of constant proportions and the law of conservation of mass ie the same number of atoms of each element must exist on the product side and reactant side of the equation. Obey the law of conservation of mass.
They must obey the Law of Conservation of Mass that states that matter cannot be created or destroyed it is conserved. There must be the same number of each atom on each side of the equation. Chemical equations have reactants to its left and products to its right.
What is the net charge on each side of the equation. Balancing the chemical equations involves the addition of stoichiometric coefficients to products and reactants. Finally a balanced equation lets up predict the amount of reactants.
Chemical equations must be balanced to satisfy the law of conservation of matter that states that matter cannot be produced or destroyed in a closed system. According to this law mass can neither be created nor be destroyed in a chemical reaction and obeying this law total mass of the elements or molecules. A chemical equation should be balanced because of law of mass conservation.
Top 4 reasons for balancing chemical equations 1. Note that if you have 1 of something it does not get a coefficient or subscript. It is important to balance chemical equations because there must be an equal number of atoms on both sides of the equation to follow the Law of the Conservation of Mass.
We want the equation to accurately represent what happens when we observe the chemical reaction in the real world. Identify each element found in the equation. This is what is represented.
It is natural that number of atoms of each element in the reactants must be balanced with the number of atoms of those elements in the products also. Qualitative vs Quantitative analysis. The number of atoms of each type of atom must be the same on each side of the equation once it has been balanced.
We know that atoms dont appear nor disappear. The chemical equation needs to be balanced so that it follows the law of conversation of massA balanced chemical equation occurs when the number of the different atoms of elements in the reactants side is equal to that of the products side. The net charge must be the same on each side of the equation once it has been balanced.
The law of conservation of mass governs the balancing of a chemical equation. You wouldnt write a maths sum as 2 2 3 so you cant write a chemical equation as H 2 O2 H 2O. Balancing chemical equations is a process of trial and error.
Balancing of a chemical equation determines how many molecules of the reactants are. Explain why we must balance all chemical equations. The word equations for a few of these reactions have been provided though most likely youll be asked to provide only the standard.
If the atom is present in a reactant compound entering the reaction it must be present in one of. The equation in which the number of atoms of all the molecules is equal on both sides of the equation is known as a balanced chemical equation. Chemical reactions must be balanced or in other words must have the same number of.
We balance equations for three primary reasons. Law of mass conservation describes that matter can neither be created nor destroyed but it can be changed from one state to another. The Law of conservation of mass governs the balancing of a chemical equation.
Chemical equations must be balanced to ensure that the number of atoms for each element is equal. The quality and quantity of the elements remain. First to reverse the effect of the wrong entry and second to record the correct entry.
Any imbalance would be a violation of the law of conservation of mass. Why must chemical equations be balanced.

Writing And Balancing Chemical Equations Introductory Chemistry Lecture Lab


No comments for "Explain Why We Must Balance All Chemical Equations"
Post a Comment